Basic Configurations and Settings for SSU
Setting up the basic configuration for a Study Start-up (SSU) includes specifying details like adding countries, IRB/ECs, essential/required documents, creating sites, and adding contacts to them. All these are the jobs of the data room administrator and need to be performed by an Administrator role team member or by your TI implementation team.
The Study Start-Up Settings allows you to set up rules that would be critical for a site activation process. These settings are global and are applicable to all sites in the room.
To configure the SSU settings, follow the steps below.
- Login into Trial Interactive entering the Username and
Password.
- Click on a room
- On the room landing page, click on the Waffle menu and select
the Settings module.
- From the left-hand navigation pane, expand the Sites settings
and select the Study Start-Up Settings.
-
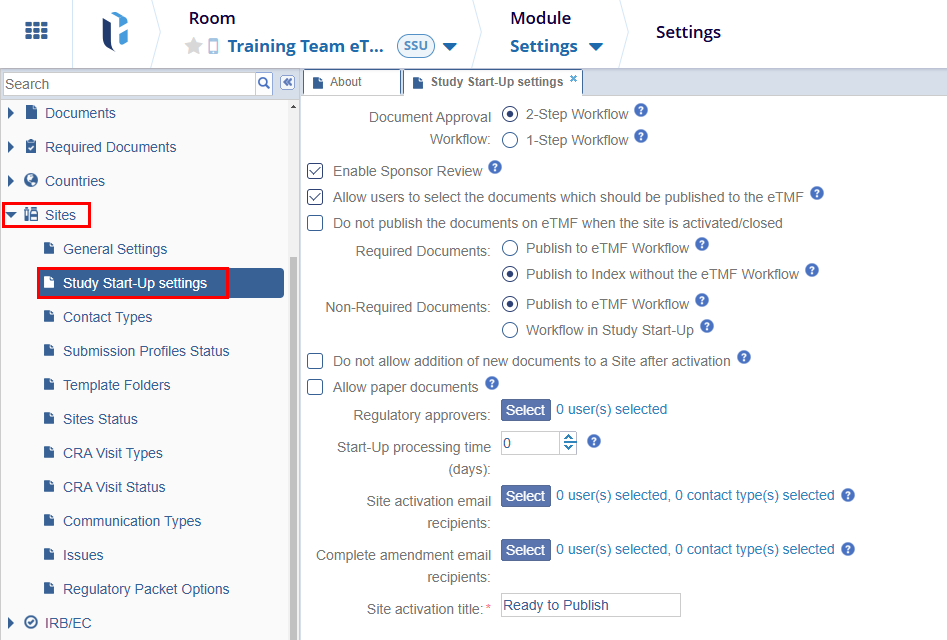
On the Study Start-UP Settings tab, configure the following settings regarding required and non-required documents on the Site Activation.
-
- Document Approval Workflow: The Document Approval Workflow allows users to select the workflow type. Select any one of the workflow types by clicking on their respective radio buttons.
- 2-Step Workflow: In this type of workflow the document should go through the two-step approval process i.e., Approve by Study Startup Specialist and Approve by Regulatory Reviewer
-
1-Step Workflow: In this type of workflow the required documents should be approved just by the Study Startup Specialist
-
-
Enable Sponsor Review: Click on the checkbox to enable the setting. If enabled documents can be submitted for additional Sponsor Review.
-
Allow users to select the documents which should be published to the eTMF: Click on the checkbox to enable the setting. If enabled it affects the site activation/non-participating process.
-
Do not publish the documents on eTMF when the site is activated/closed: : Click on the checkbox to enable the setting. If this setting is enabled, the Required and Non-Required Documents settings are disabled.
- Required Documents: Select any one of the Required Documents settings from the below options.
-
-
- Publish to eTMF Workflow: Essential Documents will be added to the default folder – Staging.
-
Publish to Index without the eTMF Workflow:
Essential Documents will be published to the eTMF Index based on
the document type auto routing
-
- Non-Required Documents: Select any one of the Non-Required Documents settings from the below options.
-
- Publish to eTMF Workflow: Non-Essential Documents will be added to the default folder – Staging.
- Workflow in Study Start-Up: Depends on the Document Approval
Workflow settings.
-
- Do not allow addition of new documents to a Site after activation: Select the checkbox to enable this setting. If enabled, no new document can be added or imported for the activated site in SSU.
- Allow paper documents: If enabled, SSU documents without attachments may be QC Reviewed. The Regulatory Reviewer can approve multiple documents.
-
-
- Configure the following additional settings.
-
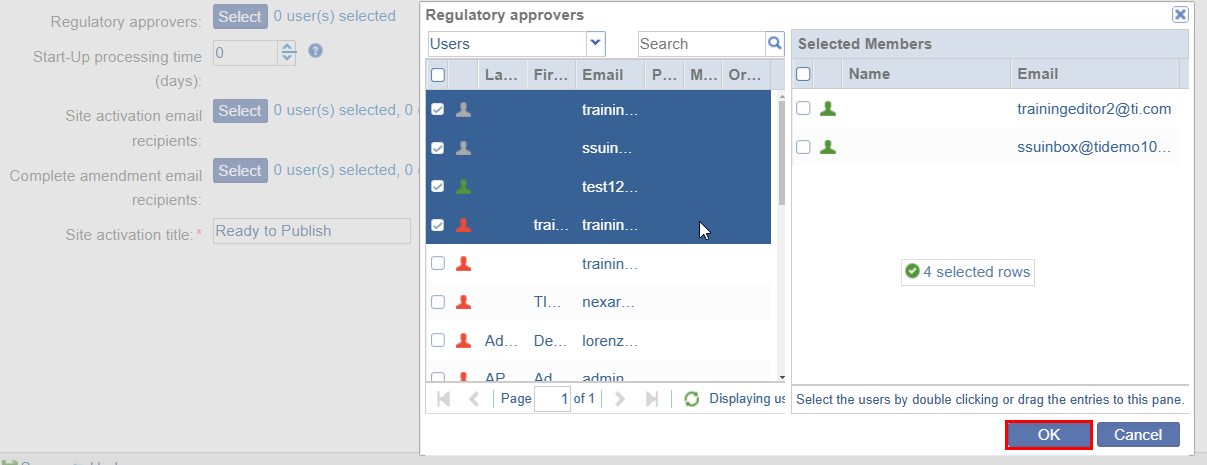
- Regulatory Approvers: To add regulatory approvers, follow the steps below
- Click on the Select button.
- On the Regulatory Approvers window, drag & drop users to
the selected members section and click on the OK button.
-
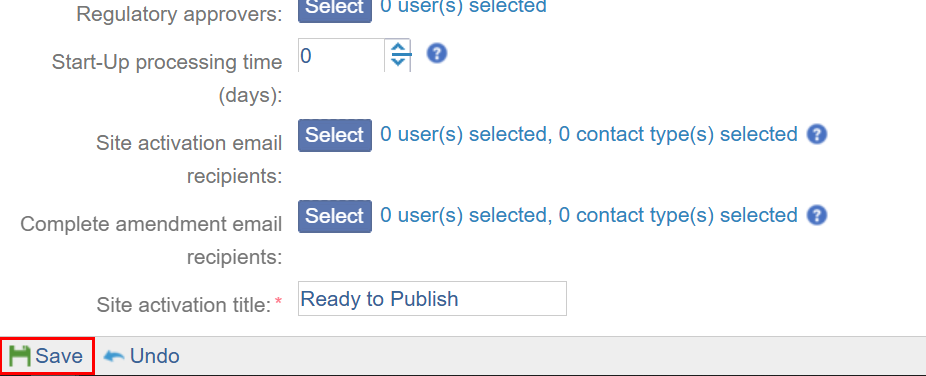
- Start-Up processing time: Adding the processing time enables having a buffer time to activate a site after the IRB/EC Approval.
- Site activation email recipients: Add recipients who will receive a site activation notification email. Follow the standard process of adding Regulatory approvers.
- Complete amendment email recipients: Add recipients who will receive a amendment completion notification email. Follow the standard process of adding Regulatory approvers.
-
Site Activation title: Mandatorily enter a site activation title.
-
-
- Once the required settings are configured, click on the Save button.