Trial Interactive eTMF Module
Know more about the Trial Interactive eTMF Application and eTMF Documents from here.
The Trial Interactive eTMF Application acts as a central access point to not only Clinical Trial Documents but also to eTMF Sites, Contacts, eTMF Completeness, and CRA Reconciliation Reports, reports in the form of Dashlets for all clinical trial activities, also to IRB Integration and Potential Sites.
You can access this module from the “Navigation Grid” in the upper-left corner of the screen. Refer to the screenshot below.
Once you enter the eTMF window, you will have access to various dashlets and can toggle between them.
In this Section, you will find the following Dashlet.
- Dashboard
- Documents
- Site
- Countries
- Contacts
- Audit Trial
- Related Documents settings
Refer to the screenshot below:
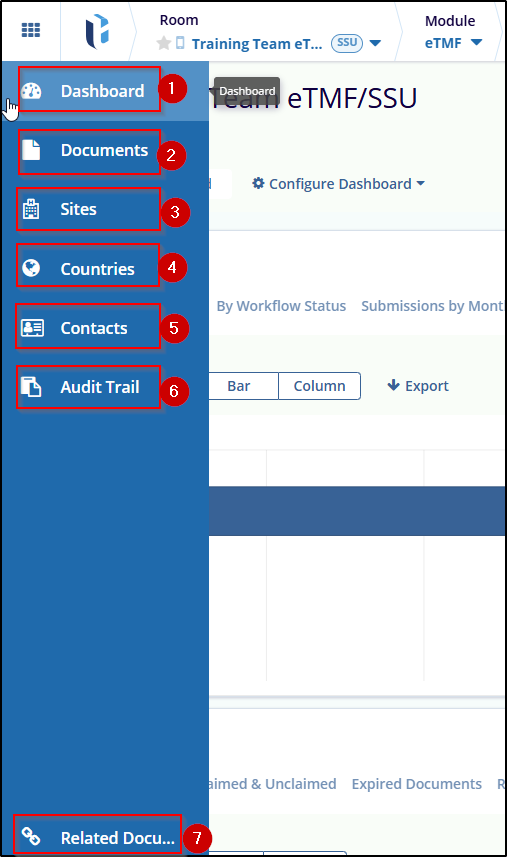
Navigate to the vertical menu at the left to choose sections. Click on the Dashboard section from the left panel.
