Steps to Site Activation
Once all essential/required documents are sent by the sites to the trial room and approved by appropriate authorities, the site can be activated. Only Site Activation Members will access the site profile to activate the site. Upon site activation, documents can be auto-named, auto-routed, and auto-filed to the appropriate location within the finalized eTMF. To note, the Administrator role can place preference on these automated setting features from the Study Start-Up settings.
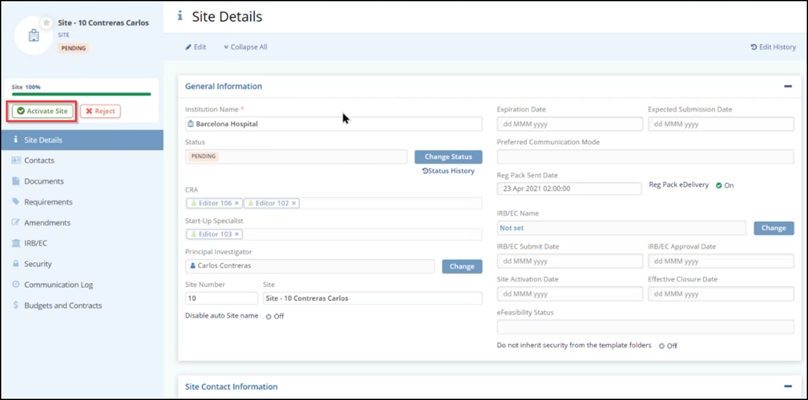
Once all the required documents are approved by both the Start-Up Specialist and the Regulatory Reviewer, the Essential Documents Progress bar shows as 100% in the Site Profile, and the Activate/Reject button at the bottom of the Site Profile dashboard appears.
To activate a site in the Trial Interactive Study Start-Up module, a series of steps must be followed.
- Select a site that displays the ‘Pending’ status. This opens the Site Details page.
- Click the Activate Site button displayed on the left side below the Country name. This opens the Activate Site popup window.
- Select the ‘Site Activation Date’ by clicking the Calendar icon.
- Select the appropriate required radio button for the documents to publish to eTMF.
- Enter the details in all the fields marked with an asterisk * in the right-side panel – Site Activation Fields.
- Click the Next button
- Review and Confirm the details in the popup window and click the Save button.
The site information and users specific to the SSU site can be imported by TI if information is provided in a formatted Excel. In addition, sites and users can be added to the site grid.
