Add Additional IRB/ECs
Although clinical trial organizations today adhere to protocols from a central IRB/EC, at times, it might be required to adhere to protocols of more than one IRB/EC. For example, an organization may have one central IRB/EC and one or more local IRB/ECs.
In the Site profile, you will be able to specify only one IRB/EC of any type. In case you need to provide additional IRB/ECs, proceed with the steps below:
- From the Grid Pane, double-click the site for which you want to specify additional IRB/ECs
- The Site Details window opens.
- Click the IRB/EC tab from the left pane. This opens the IRB/EC page.
- The top menu bar displays two options – Add Existing and Create.
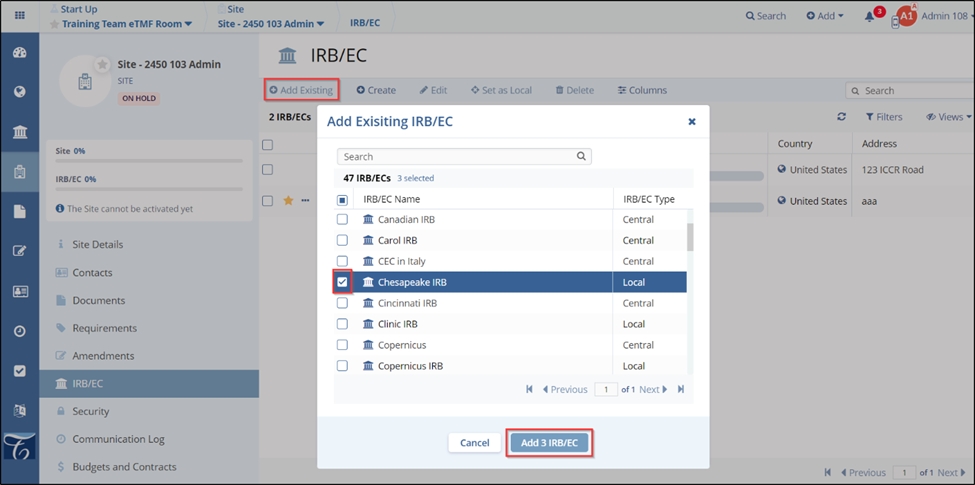
- If clicked ‘Add Existing’ – an Add Existing IRB/EC popup window opens. Select the
required checkbox(s) and click the Add IRB/EC button displayed at the bottom
of the popup window.
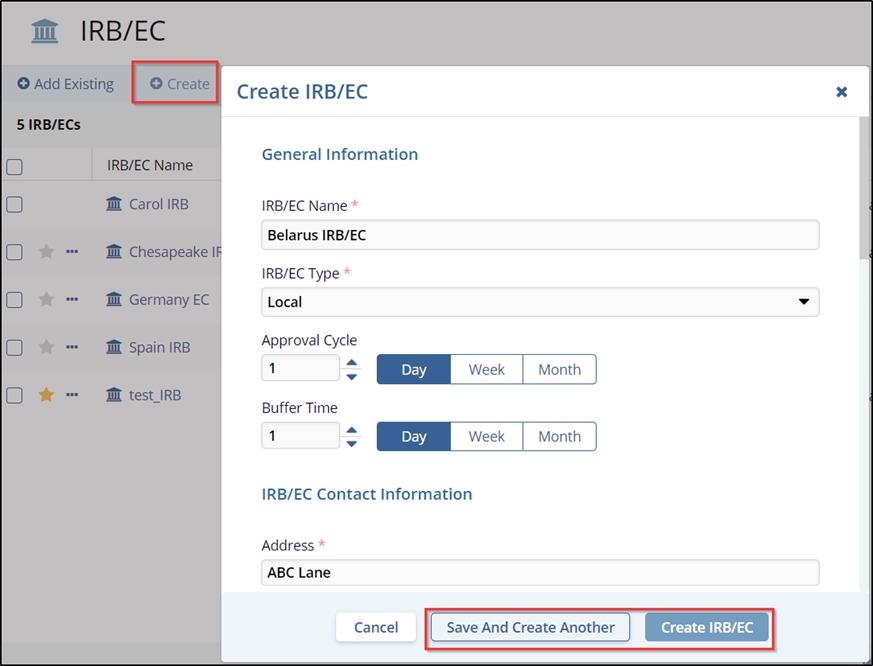
- If clicked ‘Create’ – a Create IRB/EC window opens. Enter the details in all the
fields marked with an asterisk and the other details if required. The fields marked
with an asterisk:
- IRB/EC Name
- IRB/EC Type
- Address
- City
- State
- Country
- Click the Create IRB/EC button displayed at the bottom of the popup window.
- If the user wants to create another IRB/EC at the same time, click Save and
Create Another button.
- The procedure to add from existing IRB/ECs is also the same, with the only difference being that from within the site, the Add Existing functionality will only display the IRB/ECs available in the data room.
