Submission Profile Status
It is common practice to associate health agencies with sites and send submission packages to them for their approval. Sites cannot be activated for the clinical study unless the agency's approval is received. A study may have multiple health agencies located in various countries. These agencies may have more comprehensive site activation submission packages involving hundreds of documents and that need to be reviewed and approved.
This module allows you to prepare submission profiles where the user can provide the details such as agency name, country, the status of submission, documents to be included in the submission profile, date when the package was submitted, and also the status of the submission package.
A submission package can contain documents from the eTMF, SSU, Site, Country, and IRB, or any document from the disk. For instance, the IB and protocol are already filed in the eTMF but are required for the submission package. The clinical trial organization downloads the submission package to perform QC Review as in other documents and then forwards it for regulatory review. All the actions from creating and editing submission profiles to downloading submission packages for health agencies can be performed by an admin or editor.
Through the Agency Submission section in Trial Interactive, the organization can track multiple submission packages for the same country in case one submission package is rejected. Once a site is activated, these documents are not transferred to the eTMF and are left in the submission package.
This panel provides the list of Submission Profiles and their System Statuses. The administrator may edit a submission profile by double-clicking the Display Name of a submission profile or by selecting a profile and clicking Edit from the top ribbon bar of the panel.
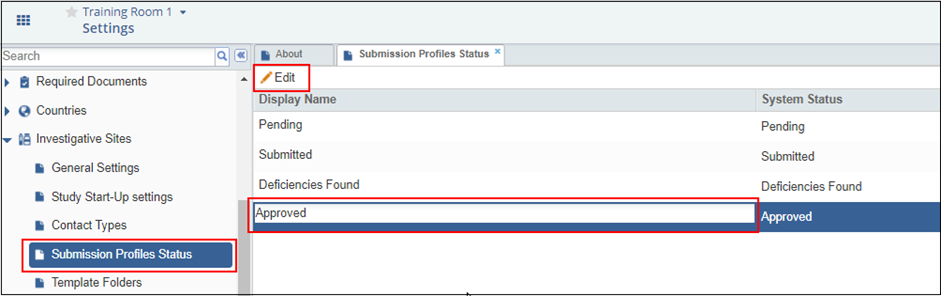 Figure 73: Submission Profile Status
Figure 73: Submission Profile Status
