Document Types and Management
In this section, we discuss creating Document Types and associated functions.
In the conduct of a clinical trial, scores if not hundreds of different kinds of documents need to be collected, categorized, and filed – some general documents, some documents that are specific to the countries in which studies are being conducted, and some documents that are specific to the investigative sites involved in the study.
All of these document types need to be set up and defined in the Trial Interactive room:
- Navigate to Navigation Grid -> Settings.
- Select Document Types from the menu on the left.
- The Document Types option expands to reveal two sub-options:
- Document Types Management and
- Common Configuration.
- Click and view each panel separately.
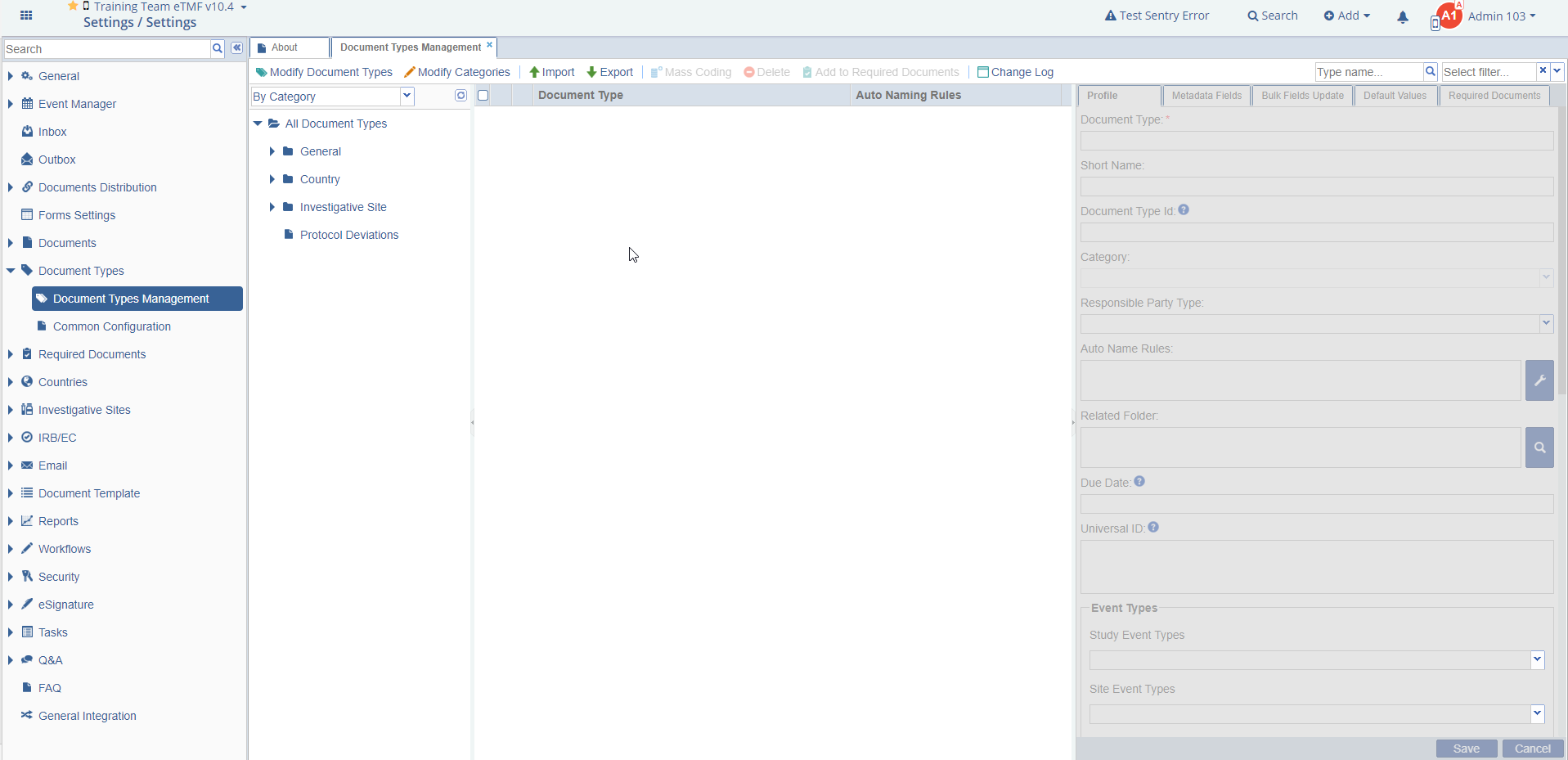 Figure 26: Document Type Management
Figure 26: Document Type Management
Click the ‘Document Types Management’ menu to open its dashboard on the right.
From this page, you can perform the various actions listed below.
- Modify Document Types
- Building the Document Type Profile
- Specifying the Related Folder
- Include Phases/Milestones
- Adding Document Types to Required Documents
- Include in Document Tracker Report
- Auto Document Type Prediction Keyword(s)
- Modifying Document Types Fields
- Default Values
