Countries
Since studies can be conducted in multiple countries across the globe, it is important for the administrator to add the countries where the clinical trial is taking, and the investigative sites located in the country during its initial setup and configuration. The following section discusses:
- Viewing and editing country profiles
- Viewing documents
Note: Adding countries, and essential/required documents to countries are discussed in
sections Adding Countries to Data
Rooms, and Setting up Required Documents for Countries respectively.
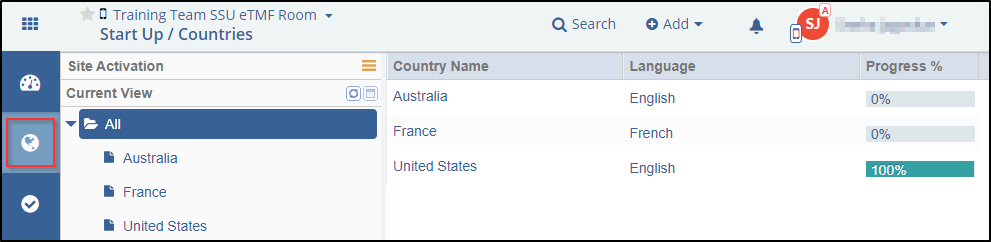
The Start-Up Countries ![]() tab accessed from the toggling menu bar on the left, allows you to set
up documents for countries associated with sites. The Countries dashboard consists of
the Current view on the left and the Grid pane on the right. Refer to the screenshot
below.
tab accessed from the toggling menu bar on the left, allows you to set
up documents for countries associated with sites. The Countries dashboard consists of
the Current view on the left and the Grid pane on the right. Refer to the screenshot
below.