Study Start Up Features
The improverd version of Trial Interactive 10.1 comprises of following features
Ability to mark sites as Closed
Support for Multiple Translations and Multiple IRB/EC Approvals
Quite often, the same Document Type requires a translated version, for example, a regulatory authority may require the document in French, German, and English. Additionally, there may be multiple IRB/ECs required in the regulatory approval workflow, and so the same Document Type may need to be recorded for each of these separate organizations. For this reason, in SSU 10.1 a document type may now be set up as required document multiple times. They can now be set up as required documents more than once based on:
• Language
• IRB/EC
This improves support for European agencies, as well as supporting the process where more than one Ethics Committee may be required for a specific Country or Site’s activation.
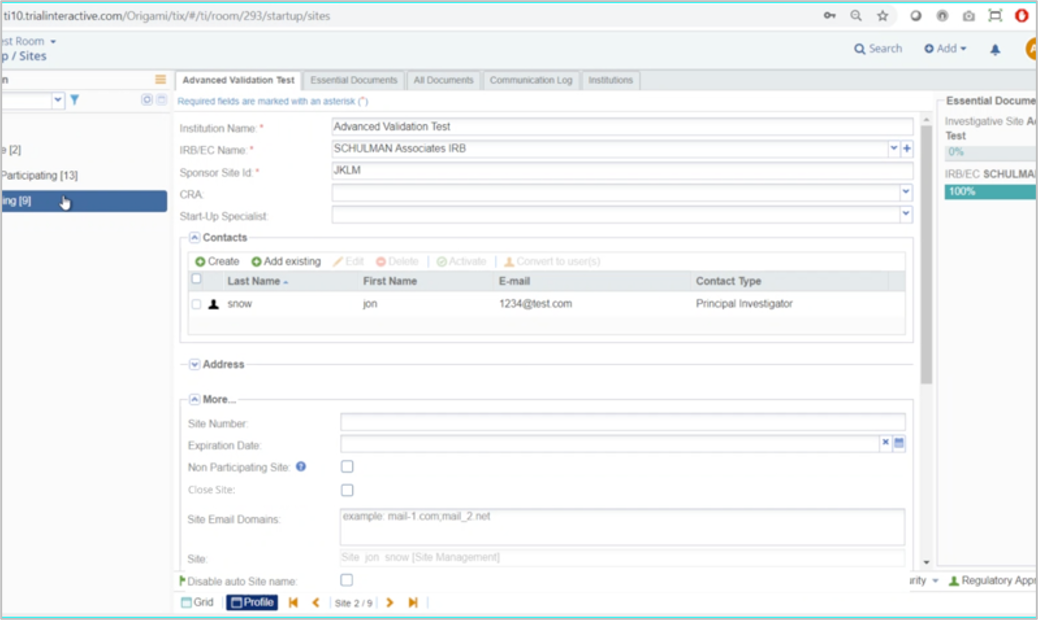
Advanced Validation
When a site is created the Sponsor/CRO may have only limited information about the site, and often cannot complete all the required fields. However, the Sponsor/CRO will ultimately gather more information that must be included before the Site can be marked Active. With SSU 10.1, Sites can now be configured to require a set of fields to be filled out with initial site creation and require additional fields that must be filled out before the site can be marked Active. This allows a simplified creation process, and at the same time ensures that all required information is collected prior to the Investigative Site going live.
Document History
During the course of Study Startup, Documents must go through a standard process before they are considered approved. With SSU 10.1, Users now have the ability to view the complete history of the documents in SSU. This includes:
• Submitted By
• Submitted Date
• Start-up Specialist Approver
• Start-up Specialist Approval Date
• Regulatory Approval Date
• Regulatory Reviewer
