Platform Features
The improved version of Trial Interactive 10.1 comprises of following Platform features
Multi-tenant SSO
Introduced in 10.1 is support for SAML Federated Single Sign-On for the TI multi-tenant platform. Now, all customer domains on multi-tenant can be configured with single sign-on through their own Corporate Directory, as well as third party IAM providers.

- You can drag and drop email messages from MS Outlook into TI and the email will import along with all document attachments. Once in TI, MSG files may be converted to PDF.
- You can drag and drop individual document attachments from an email into TI and the documents will import.
- You can drag and drop a single document or email message onto a folder or placeholder and it will auto-classify.
- You can drag and drop many documents or email messages into the import modal in TI and they will all load into the import box.
Certain browsers such as Mozilla Firefox™ require an Outlook plug-in to fully support this feature. Chrome, Edge, and IE 11 do not require a plug-in. Links to download the required plug-ins will be provided in the online help.
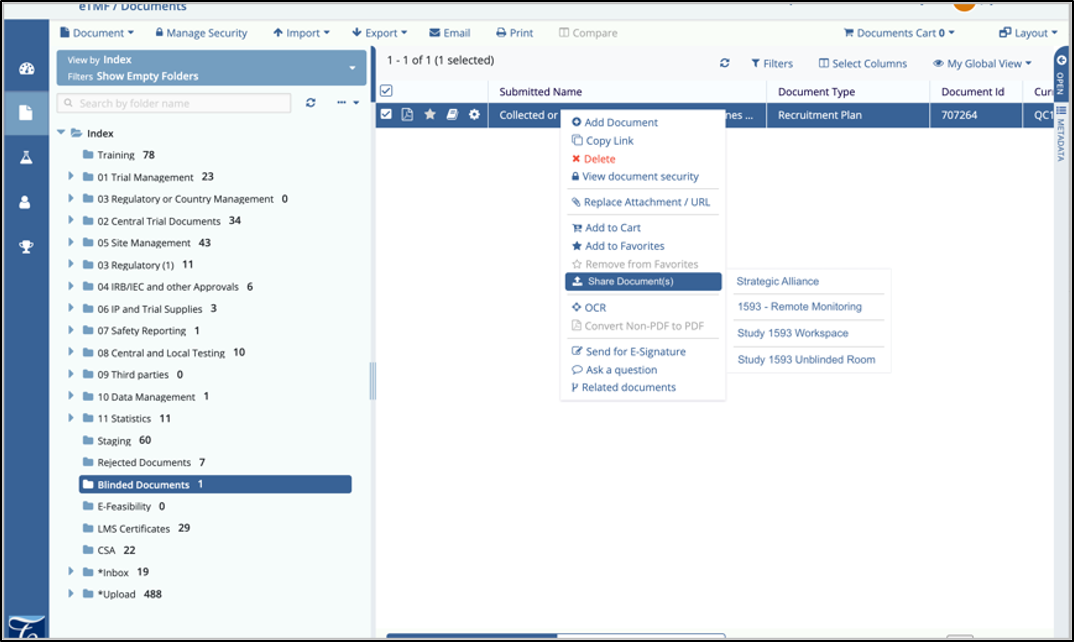
CROSS-ROOM DOCUMENT SHARING
Content Management in life sciences is a very complex set of relationships that happen across multiple repositories of content, owned by different business units. Clinical needs content management and a TMF system that authors and captures content generated during the planning and execution of a Clinical Trial. Other repositories exist that are owned by Quality, Regulatory, R&D, Commercial, Training, Medical Writing, and many others. Finally, quite often, Clinical needs to keep unblinded content in a separate repository as well as the ability to access site documents from remote monitoring workspaces.
Each of these units broadly has its own workflows, approval cycles, and policies for the management of content. They also have separate owners who want control of the content management and metadata policies within. When it comes to a Clinical Trial, much of this content is archived and ends up in the Trial Master File.
10.1 introduces the capability to share documents easily, in a controlled fashion, between TI repositories called Rooms. This sharing is configured by room and may be enabled to many rooms, to allow for multiple content sharing scenarios, including: Sharing a single document to many eTMFs, sharing from many sites to a single eTMF, and sharing from an eTMF to other groups.The 'chain of custody' of these documents is very important to maintain. As approved document versions are shared between rooms, the document source location and destination are tracked and can be viewed. Metadata that is common between two rooms will be shared along with the content, and only users with permission may share content. However, users may not share content to rooms to which they do not have access, like the eTMF for example, to enforce a controlled document workflow between repositories.