How to Regulatory Review a Document with Attachments
Once the regulatory reviewer receives an email stating about a pending review, he/she logs into TI to locate the documents waiting for review in the Regulatory Review section.
Steps to Regulatory Review a Document
- The easiest way to find out all the documents in the study start-up waiting for
regulatory review is to activate the By Regulatory Approval Status view
from the left panel which lists out all the documents in the SSU pending for
approval. Refer to the screenshot below.
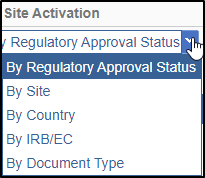
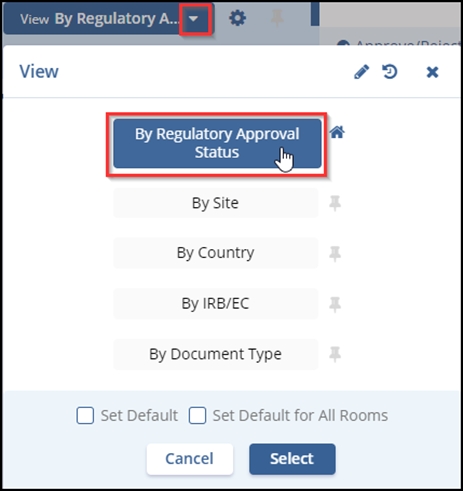
- Select a check box of a document that displays the Pending status.
- Click the activated Document button displayed at the left bottom of the screen. OR
- Click on the PDF
 icon.
icon. - The document opens in the centre of the dashboard. Refer to the screenshot
below.
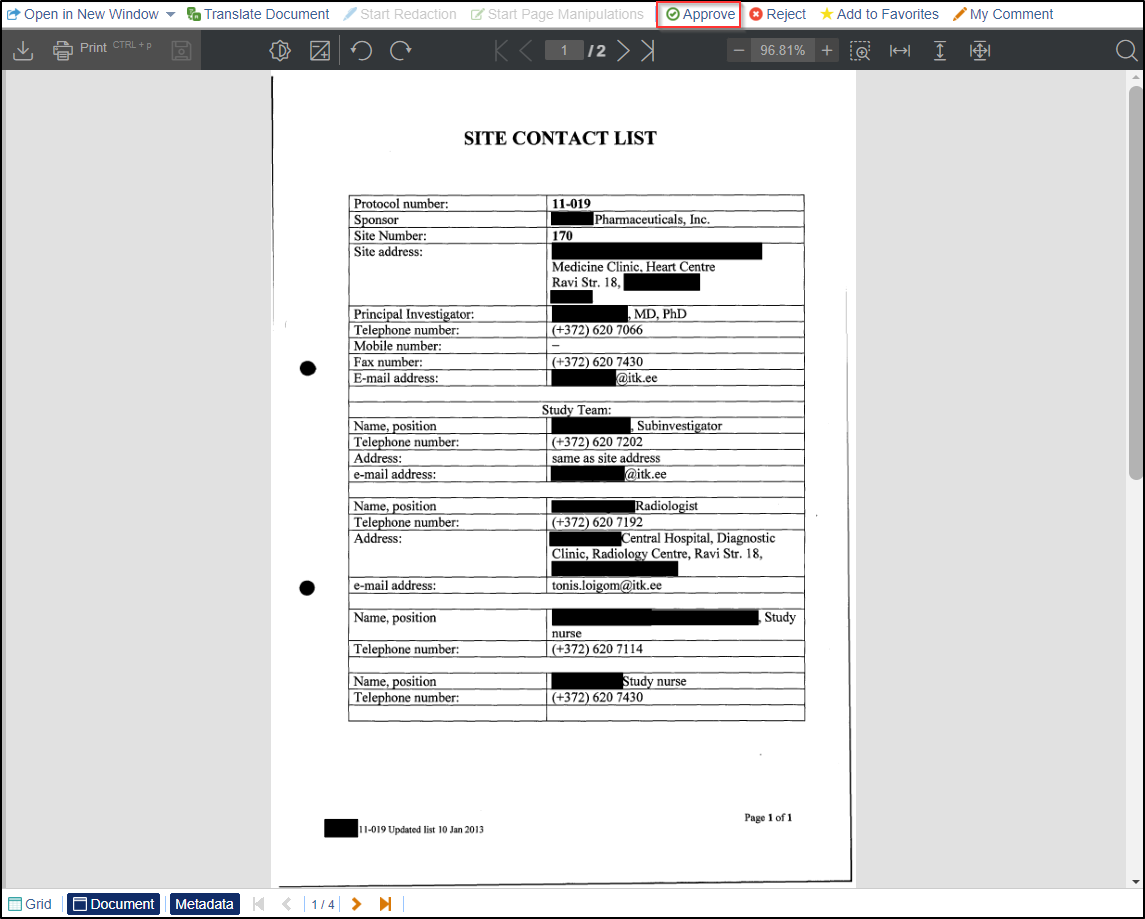
- Read the document. If the document is appropriate, click the Approve
button displayed at the top menu bar. Refer to the screenshot below.
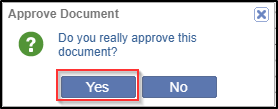
- An Approve Document pop up window is displayed to confirm to approve the
document. Click Yes. Refer to the screenshot below.
- If you want to reject the document, Click the Reject button displayed at
the top menu bar. Refer to the screenshot below.
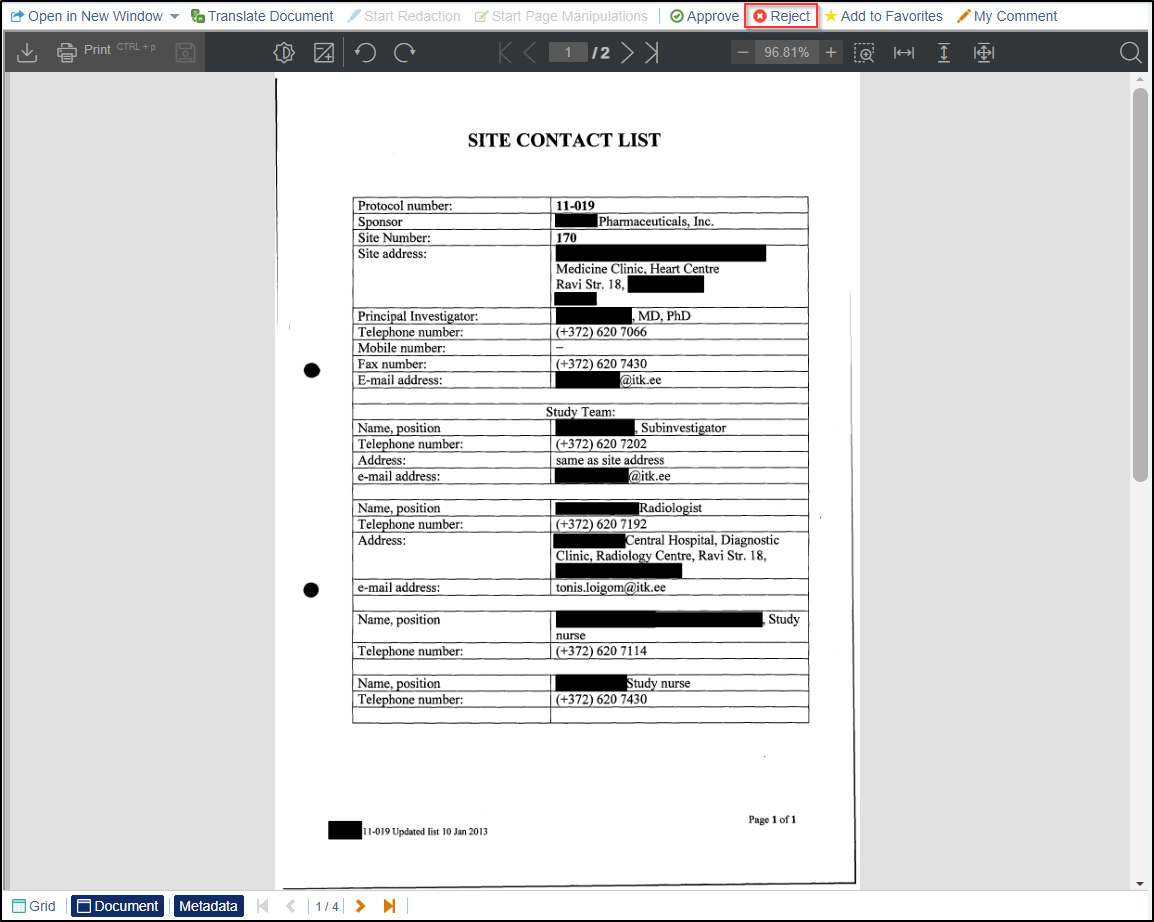
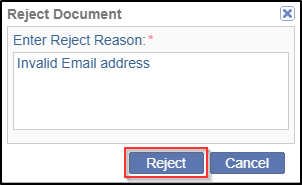
- A Reject Document pop up window is displayed to manually enter a reason for rejecting the document.
- Click the Reject button in the Reject Document pop up window. Refer to
the screenshot below.
Depending upon the decision taken, a message will pop up indicating that the change is now committed to the database. The document is now locked and cannot be opened for review again. The Regulatory Approval Status of the documents throughout the SSU will now reflect the appropriate status from Pending to Approved, or Rejected. The Document button at the bottom of the dashboard, and the Approve or Reject buttons are now disabled to prevent further changes.
