Document Types and Management
In this section, we discuss creating Document Types and various functionalities related to it.
In the conduct of a clinical trial, scores if not hundreds of different kinds of documents need to be collected, categorized, and filed – some general documents, some documents that are specific to the countries in which studies are being conducted, and some documents that are specific to the investigative sites involved in the study.
All of these document types need to be set up and defined in the Trial Interactive
room:
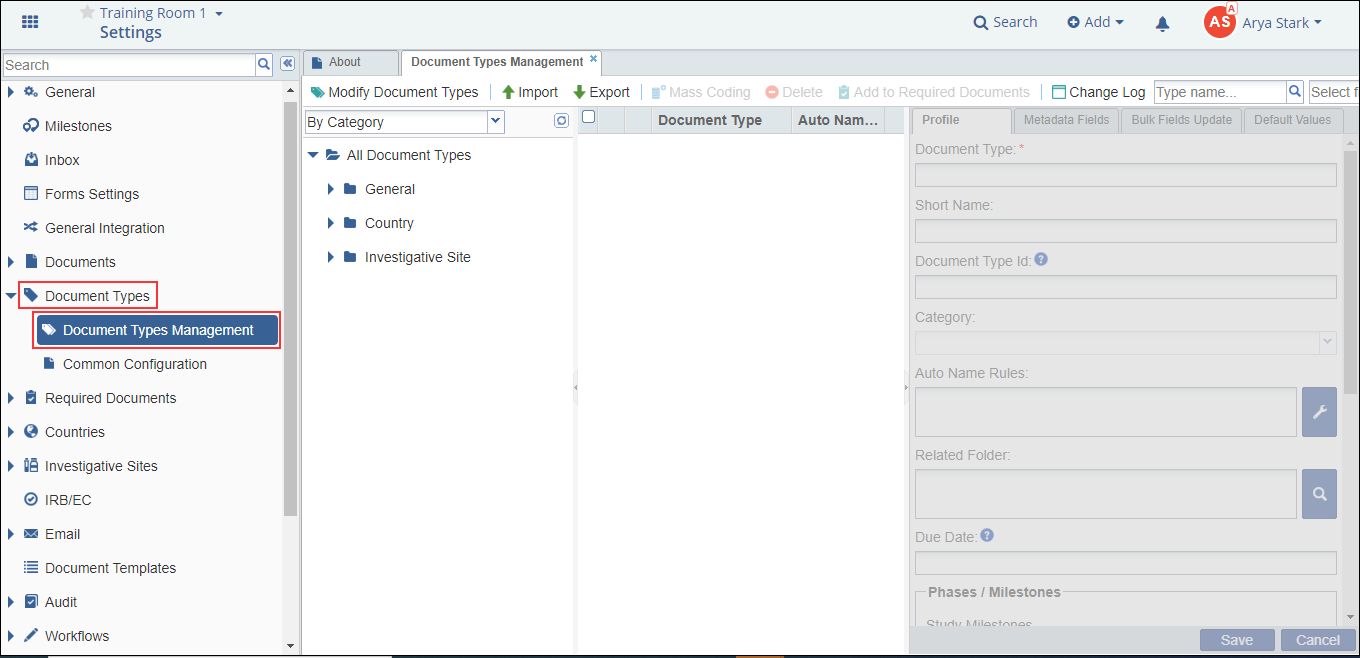
- Navigate to Main Navigation -> Settings. The Room Settings page opens.
- Select Document Types from the menu on the left.
- The Document Types option expands to reveal two sub-options:
- The Document Types Management and
- The Common Configuration.
- Click and view each panel separately.
Refer to the screenshot below:
The Documents under the Document Types created from here can be viewed under the By Document Type view.
Each view or panel are discussed in separate topics accessible from the left pane of this help:
- Document Types Management
- Common Configuration
