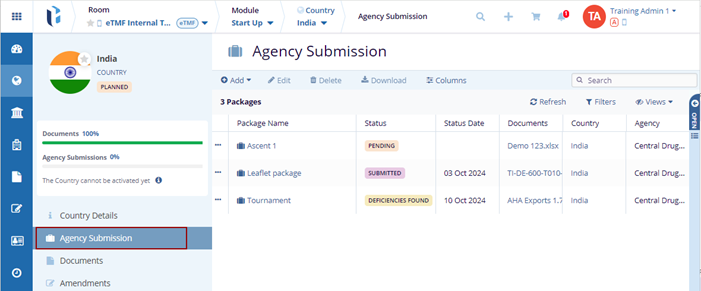
Agency Submissions
The Agency Submission module streamlines the approval process for activating clinical study sites. Users can create submission profiles, specifying agency details, submission status, required documents, and submission dates. Packages can include documents from various sources (eTMF, SSU, Site, Country, IRB, or disk). Admins and editors manage profiles, perform QC reviews, and track multiple submissions per country. If a package is rejected, new submissions can be made. Approved documents remain in the submission package but are not transferred to the eTMF.
Defining Health Agencies
Health agencies must be defined at the domain level before adding submission profiles and downloading packages. These agencies are then associated with sites.
Note: Contact the help desk to create Health Agencies if they are not already created.