Site
The Sites Module in the Study Start-Up (SSU) process refers to the set of activities involved in identifying, evaluating, selecting, and activating clinical trial sites.
Clicking the Sites tab from the vertical menu bar leads you to the Sites section. This is where the Start-up Specialists will perform their functions, and the users of the sites are allowed to submit and approve documents specific to the sites.
Views:
Users can customize views as needed, and the grid view adjusts accordingly to display relevant information.
- On the left side of the Sites screen in the index panel click the View By drop-down arrow.
- The View pop-up window is displayed with the views.
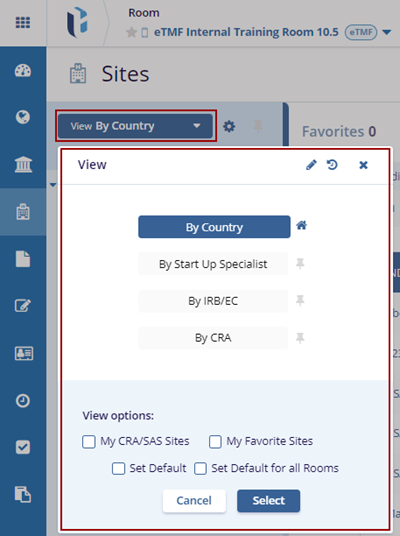
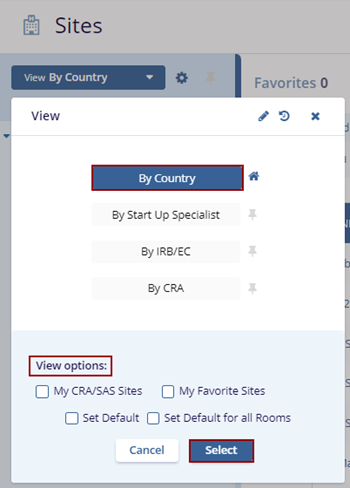
- Select the By Country view, optionally check the desired View Options at the bottom of
the View pop-up, and click the Select button.
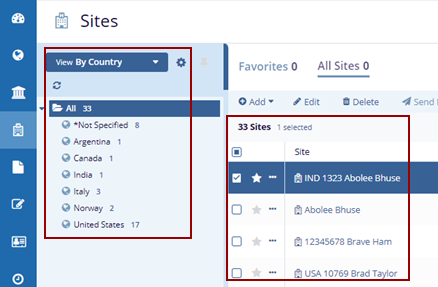
- The Index panel displays the Country list and the associated sites on the right side.
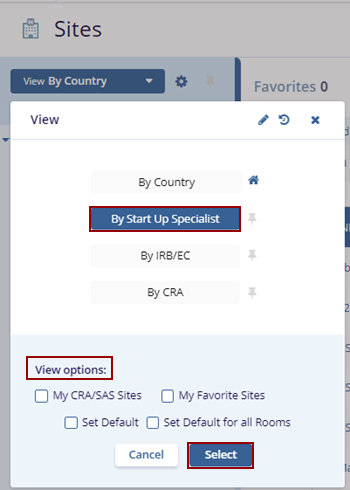
- Select the By Start Up Specialist view, optionally check the desired ‘View Options’ at
the bottom of the View pop-up, and click the Select button.
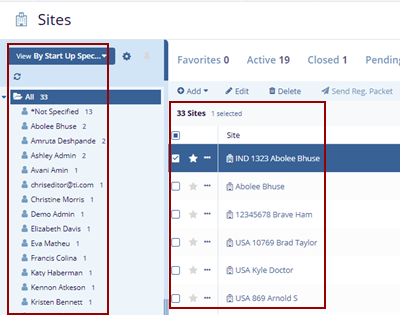
- The Index panel displays the Start Up Specialist Users list and sites on the
right side.
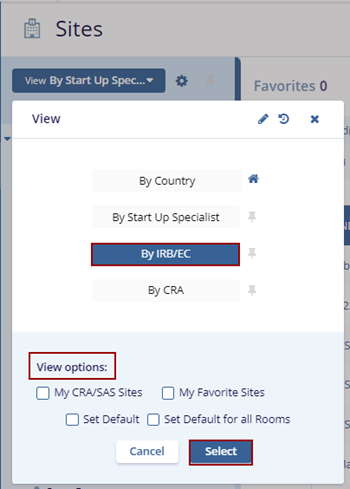
- Select the By IRB/EC view, optionally check the desired ‘View Options’ at the bottom of
the View pop-up, and click the Select button.
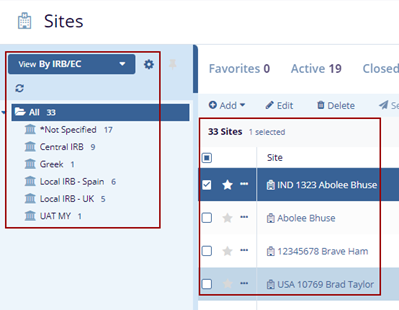
- The Index panel displays the IRB/ECs list and sites on the right side.
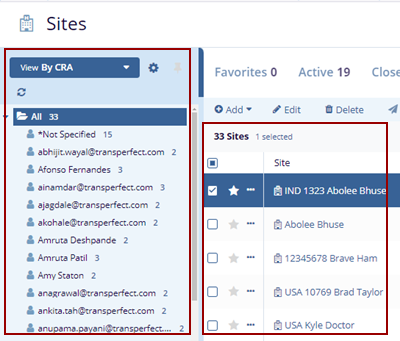
- Select the By CRA view, optionally check the desired ‘View Options’ at the bottom of the
View pop-up, and click the Select button.
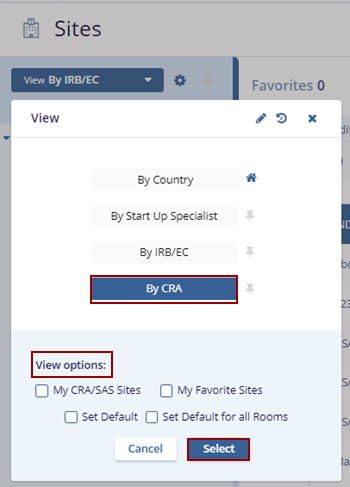
- The Index panel displays the CRA Users list and sites on the right side.