IRB/EC
Clinical trial sites must follow IRB/EC protocols to ensure efficient and effective operations. While organizations are encouraged to use central IRBs over multiple local IRBs, the choice depends on the research enterprise, particularly for multi-site trials.
Trial Interactive accommodates both central and local IRBs, offering flexibility for multi-site trials. While a single central IRB is recommended, multiple IRBs/ECs may be necessary. Trial Interactive enables users to add and specify IRB/ECs as needed.
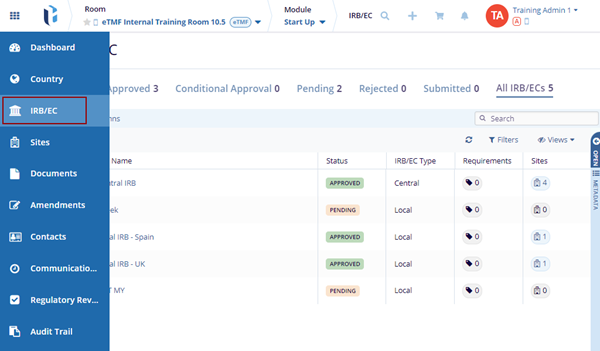
Clicking the IRB/EC tab from the toggling menu bar on the left lists the IRB/ECs available to the room. The IRB/EC dashboard consists of the Grid pane in the center with the following columns to view and edit:
- IRB/EC Name
- Status – Displays if the IRB/EC is Approved, Submitted, Pending, or Conditional Approval.
- IRB/EC Type – Displays if the IRB/EC is Central or Local.
- Progress – Displays the percentage of required documents collected for each IRB.
- Sites – Displays the number of sites linked to the IRB/EC.
- Address – Displays the address of the IRB/EC.
