Adding or Creating New IRB/ECs
To add IRB/ECs as required by sites, the administrator needs to do the following:
- Assuming that you are in the room Settings section, click IRB/EC from the left panel.
- The IRB/EC Management window opens in the right side panel. Refer to the screenshot
below.
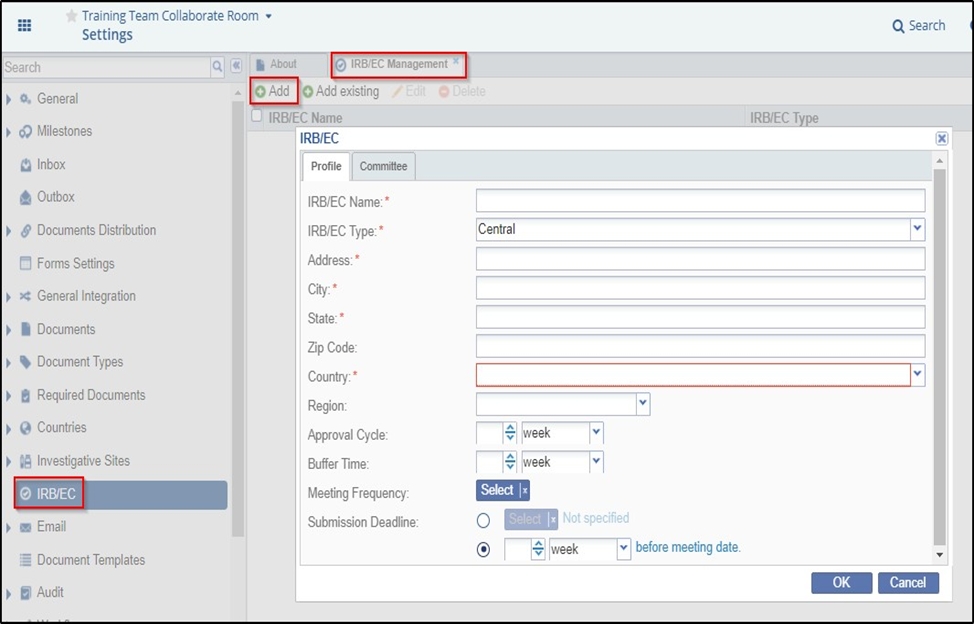
- Click Add in the top menu bar from the IRB/EC Management window.
- This opens a blank IRB/EC Profile form to fill in the data. Enter all the
details as applicable. Note that the fields maked with red asterisk (*) are
required. Refer to the screenshot below. Some of the important fields of IRB/EC window are discussed below:
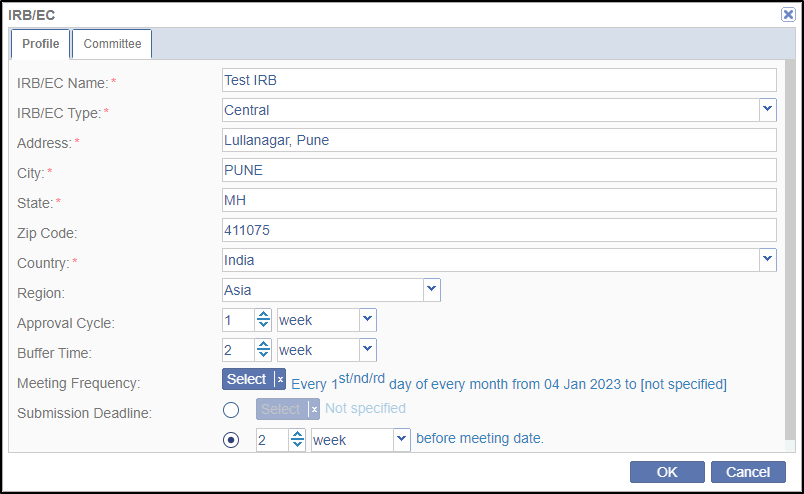
- Approval Cycle: This is the IRB/EC’s processing time and can be denoted in days/weeks/months.
- Buffer Time: This is the extra time IRB/EC may need to approve the documents.
- Meeting Frequency: This denotes the meeting frequency of
the IRB/EC which could be daily/weekly/monthly/yearly.
- Click the Select button to open the Meeting Frequency popup window. Select a required radio button for Frequency and the required appropriate option(s) from the right pane related to the Frequency.
- Select the Start and End Date from the
calendar
 icon for Range of Recurrence section. Click OK to
save the changes. Refer to the screenshot below.
icon for Range of Recurrence section. Click OK to
save the changes. Refer to the screenshot below.
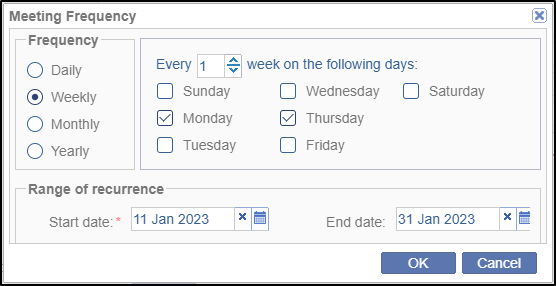
Note: The right pane is dynamic as per the option selected in the Frequency section. - Submission Deadline: This denotes the submission deadline in day/week/month/year before theIRB Meeting Date.
- Click the Committee tab in the popup. This allows you to add the
details of Ethics Committee associated with the IRB. Refer to the screenshot
below.
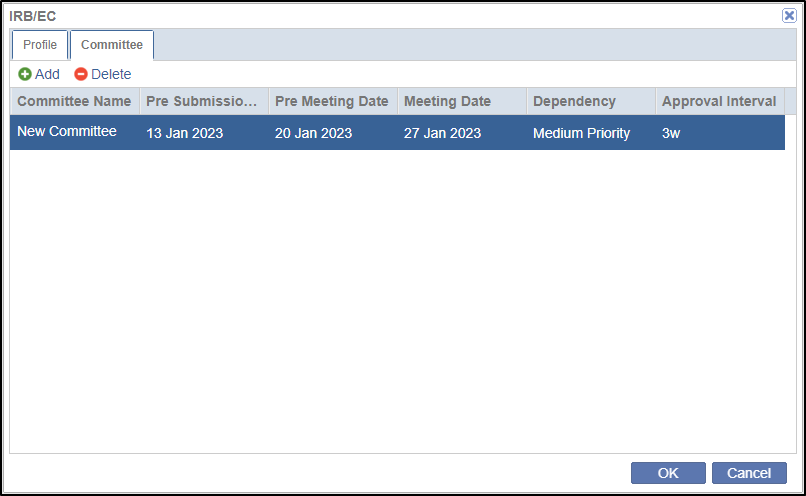
- Click Add in the Committee tab. This will add a new row to add an ethics committee by the name of ‘New Committee’.
- Double-click each field in the row to enter the details for the committee.
- Click Ok.
- This opens a blank IRB/EC Profile form to fill in the data. Enter all the
details as applicable. Note that the fields maked with red asterisk (*) are
required. Refer to the screenshot below.
- This will add the IRB/EC to the study and also to the centralized IRB library.
- Repeat the process for as many IRB/ECs as required.
