Documents View
The Trial Interactive eTMF Documents is the central repository for all the clinical trial documents in their original digital format with Digital Signatures wherever applicable, records, or documents converted from one format to another like paper documents, images converted to PDFs, besides videos and recordings pertaining to trials.
Here, you can configure and store trial master file 'essential documents' pertaining to clinical trials, view and edit documents attachments, manage security privileges on them, import and export documents and their metadata, mail them to other users besides many others.
To comply with eTMF Completeness, you can track the progress right from documents collection to the finalization of a document through Milestones and assignments of Tasks to authorized personnel. Besides, this application also provides you with the facility to post Questions and Answers along with the generation of FAQs for further insight.
The documents are then subjected to Quality Control, and Quality Review checks as specified by the FDA.
You can access the Documents View by clicking the Documents icon from the menu bar at the left of the dashboard. Refer to the screenshot below:
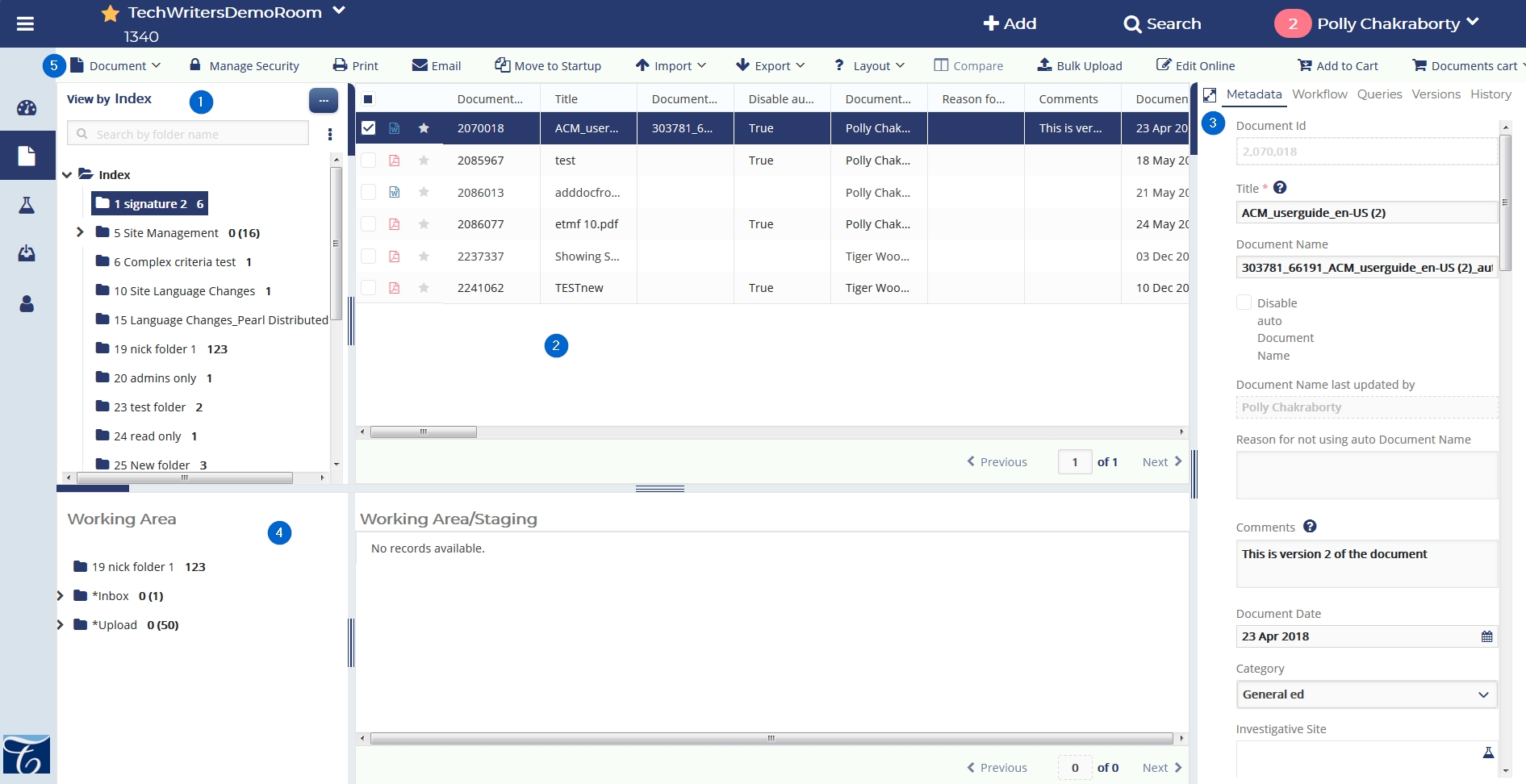
Refer to the table below for more description on each numbered part.
| No. | Part Name | Description |
|---|---|---|
| 1. | The Room Index | The Room Index consists of folders organized into a tree-like structure starting with Index as the root folder. |
| 2. | The Documents Grid | Select a chid folder from the Index to populate and view its documents in the Documents Grid. |
| 3. | The Document Data Panel | Tick a checkbox next to a document in the Documents Grid to populate the Document Data Panel. |
| 4. | The Working Area | Shows the record of the document currently working in. |
| 5. | The Top Ribbon Bar | Access various functionalities required for eTMF operations from here. |
Click the links in the table below for more details on each part or section.
