SSU User Interface
The SSU Module in Trial Interactive (TI) provides a streamlined platform for creating, monitoring, and activating clinical trial sites. Users can add, upload, track, and review the necessary documents for site activation through this interface.
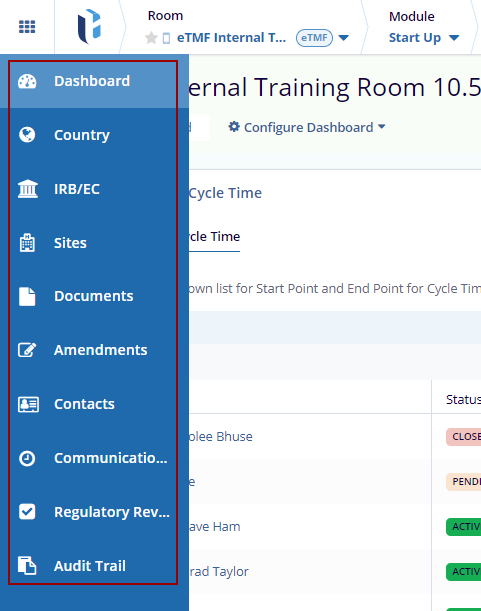
The Start-Up module includes the following sections:
- Dashboard
- Country
- IRB/EC
- Sites
- Documents
- Amendments
- Contacts
- Communication Log
- Regulatory Review
- Audit Trail
Note: The Toolbar options are available per the room settings and user access.
