Amendments
The Amendments refer to modifications or updates made to study documents, protocols, contracts, or regulatory submissions after the initial setup but before the study begins or progresses to the next phase. These amendments typically arise due to regulatory requirements, sponsor requests, site-specific needs, or protocol changes.
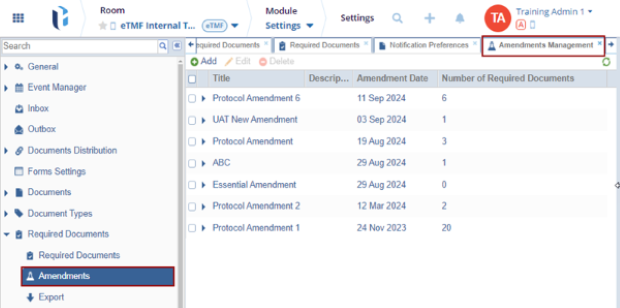
To Create an Amendment, follow the steps below.
- Click Add, the Create Amendment window is displayed.
-
Fill in all the required fields.
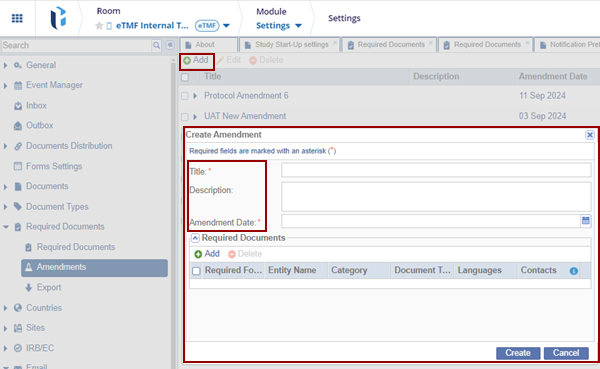
- Click Add, within the Create Amendment window to add the equired Documents.
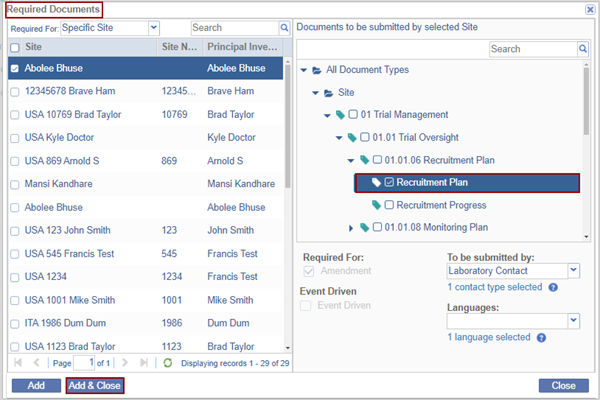
- The Required Documents screen is displayed.
- Expand the Required For field to view the options. Select the required option from the
list.
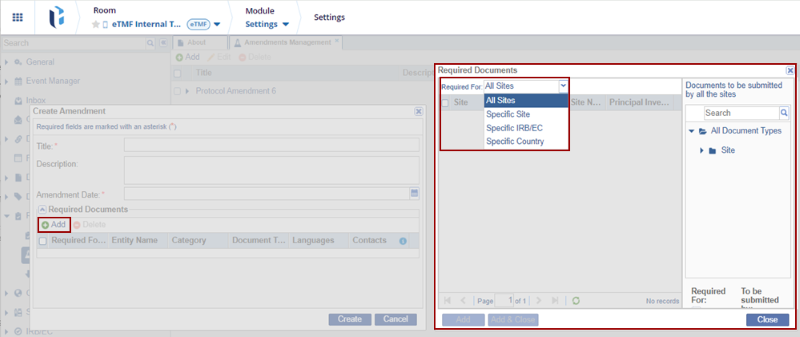
- The list of the Sites is displayed on the left pane.
- On the right pane select the documents to be submitted by the selected Site and fill in the required fields.
- Click Add & Close.
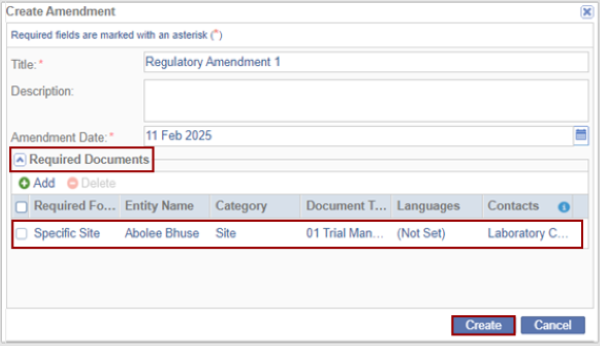
- The added Required Documents are displayed within the Required Documents section on
the Create Amendment window.
-
Click Create. The create Amendment is displayed in the grid.
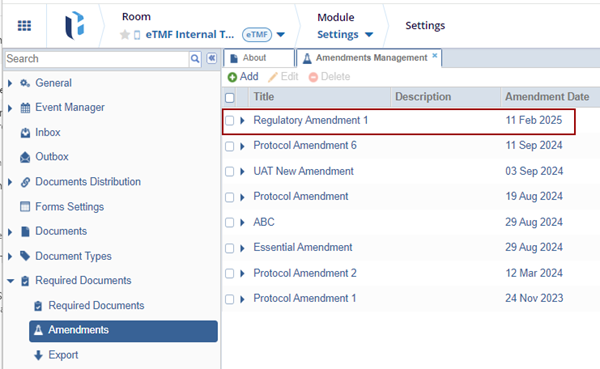
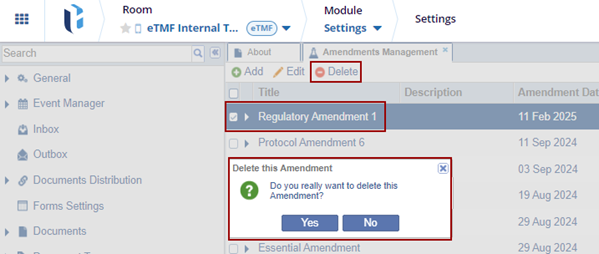
To Edit and Delete the created amendment, follow the below steps:
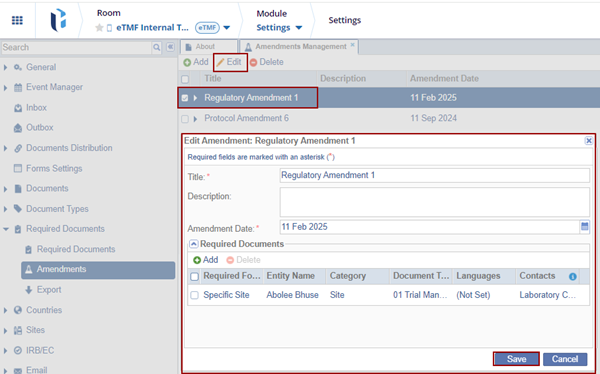
- Locate the amendment and click the Edit button.
-
The Edit Amendment window is displayed. Make the required changes and click Save.
- Locate the amendment and click the Delete button.
-
The Delete this Amendment dialog box is displayed. Click Yes if the user wants to delete the amendment.